Date published: 5 September 2024
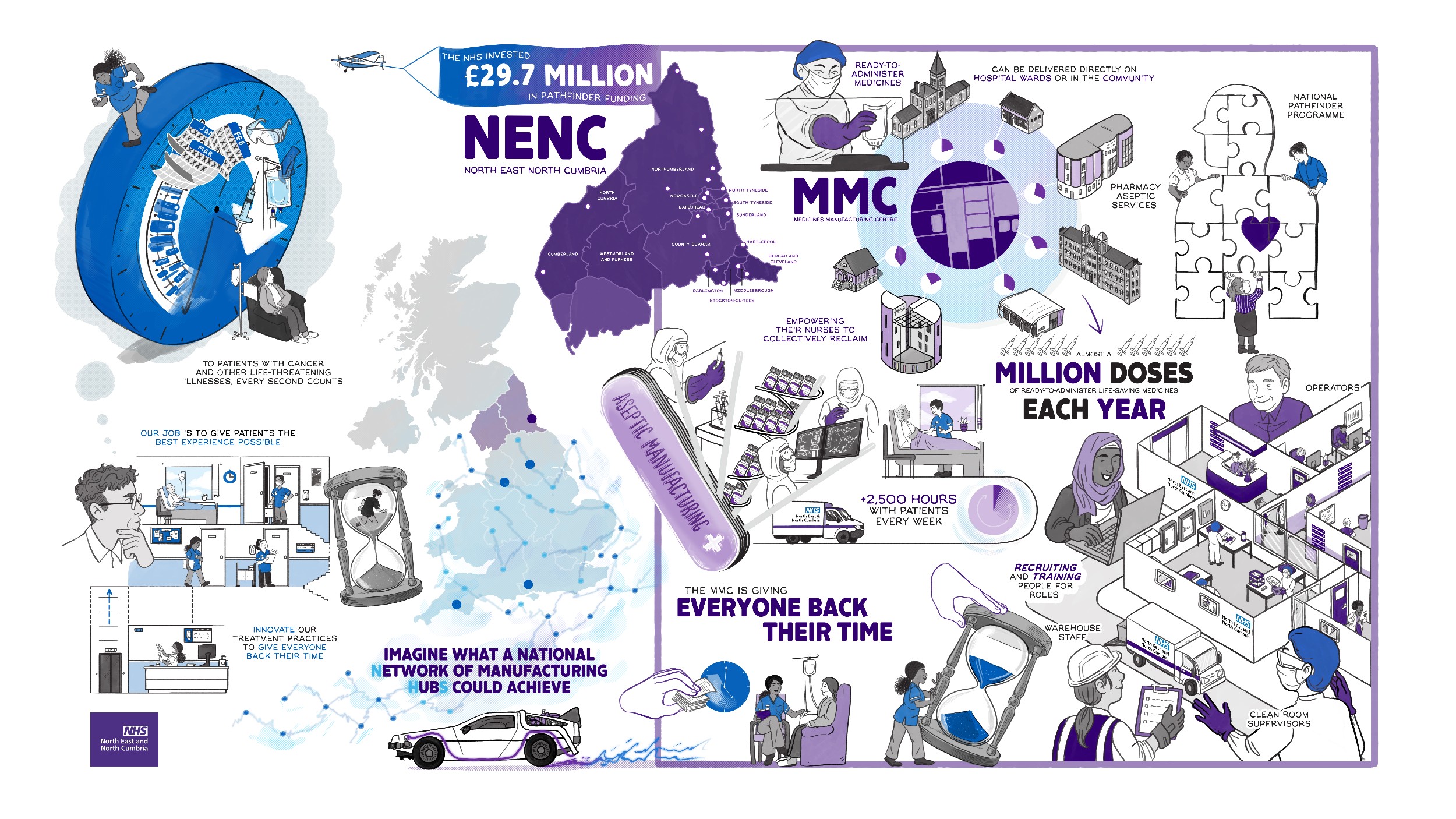
Plans for a new flagship NHS Medicines Manufacturing Centre to serve patients across the North East and North Cumbria, have been approved by NHS England and the Department of Health and Social Care.
Following a meeting of the Joint Investment Sub-Committee (JISC), plans for the new regional facility got the green light. NHS leaders, working together through the North East and North Cumbria Provider Collaborative, secured the £29.7 million of national NHS funding thanks to a successful joint bid between the region’s NHS Foundation Trusts back in November 2022.
The manufacture of sterile medicines in the NHS plays a vital, but often unseen part in the delivery of safe and high-quality patient care. Known as ‘aseptic’ services, NHS Foundation Trusts across the region already have their own production units but these are all working at, or nearing, capacity.
The new NHS Medicines Manufacturing Centre will serve the region’s entire hospital network and support existing aseptic units. It will produce large volumes of chemotherapy treatment, as well as other ‘ready to administer’ injectable medicines, including intravenous antibiotics. It will also manufacture ‘pre-labelled’ medicines to help support local hospital teams as patients are discharged home.
Based at Seaton Delaval, the new facility will work in a hub and spoke model alongside existing aseptic units in the region’s hospitals and aims to greatly increase capacity. It will safeguard the supply of vital drugs for patients in the region for the next 20 years by creating an in-house and sustainable supply chain within the NHS.
Once the new regional facility is up and running, it will release capacity in local hospital units allowing them to focus on more complex, bespoke medicines close to patients. There will also be major benefits for staff and patients, by freeing up valuable nursing time on hospital wards to allow staff to provide other clinical care, rather than having to prepare injectable medicines themselves.
The £29.7million cash injection for the region’s NHS comes following a national review of NHS pharmacy aseptic services which took place in 2020. It is part of £75million allocated to NHS England’s Infusions and Special Medicines Programme to develop a number of pathfinder hub sites across the country for the production of aseptic medicines. The North East and North Cumbria Medicines Manufacturing Centre is the largest of these sites representing a significant benefit to the region.
Mr Ken Bremner MBE is Chair of the region’s Provider Collaborative* and Chief Executive of South Tyneside and Sunderland NHS Foundation Trust. He said:
“We’re delighted to get the go-ahead and start building work on this new NHS facility.
“Our existing aseptic units across the region will continue to play a vital role, but this new facility now means we can achieve much better manufacturing efficiencies by working on a much bigger scale. It will allow us to greatly increase our capacity for medicines production, create a sustainable model for the future and help us save time and money that we can reinvest in patient care.
“To secure this level of national investment for the region is testament to our collaborative approach and the collective efforts of many colleagues. As works gets underway, it will also bring many wider benefits to the region, with the creation of around 150 new jobs which will generate economic growth.”
Samantha Allen, Chief Executive of the Integrated Care Board (ICB) for the North East and North Cumbria, said:
“We are really delighted to see this project coming to fruition thanks to the collective efforts of the region’s Provider Collaborative.
“Making sure we have our own supply of ready-made injectable medicines manufactured in-house by the NHS, for the NHS, is great news for patients. We know pressure in this area will only increase as medicines and treatments continue to advance and this will futureproof the supply chain for many years to come for people here in our region. It really is a major feat for health and care in the region and great news for our overall economy.”
Following a competitive tendering process, local construction company Merit have been appointed to design and ‘fit out’ the new facility, whilst specialist supplier, Getinge, will provide the high-tech isolator equipment used to manufacture the medicines.
Work is ongoing with Merit to finalise the detailed design and with Getinge, who will supply the specialist isolator equipment. Building work will start on site in Seaton Delaval this autumn.
Tony Wells, CEO at Merit, North-East based specialist off-site construction firm, said:
“We are looking forward to getting started on this exciting project and using our vast experience in the design of sterile manufacturing facilities. Merit’s FLEXI POD solution is ideal for this project, bringing enormous advantages in terms of speed, quality, a sustainable centric design and affordability.”
Jonathan Grogan, Getinge Life Science National Sales Manager for the UK and Ireland, said:
“Our Isolators are a critical part of any sterile manufacturing environment and we are delighted to have been appointed as the supplier for this important new NHS facility. We look forward to our continued Partnership with Merit and the project team in our Passion for Life.”
The region’s NHS plans to start operating from the new Medicines Manufacturing Centre in the back end of 2026 once full approval is granted from the Medicines and Healthcare products Regulatory Agency (MHRA).